Nitric Acid
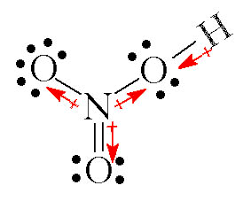
Nitric acid (HNO3) is a chemical compound in the form of a colorless or white, transparent liquid with acidic properties. This acid is formed from a mixture of nitrogen, sodium and hydrogen.
it is one of the strongest acids with the strongest acid properties among common acids. This acid has the ability to produce hydrogen ions (H+) in the solution and therefore has strong acidic properties.
this matter also reacts with strong metals and many non-metals and organic and aliphatic compounds.
Application of nitric acid
Nitric acid 55% has many different uses, which can be mentioned as follows:
Production of chemicals such as nitroglycerin, nitrocellulose and various nitrates
Use in chemical industries to produce fertilizers, raw materials for dyeing industries, alkalis and all kinds of plastics
it is used as electronic industries as a charge transfer material in the manufacture of transistors and electronic circuits
and laboratories as a chemical substance for testing and testing chemical substances and compounds
Use in mining industry as a solvent to extract metals from minerals
Used in the textile industry for dyeing and polishing fabrics
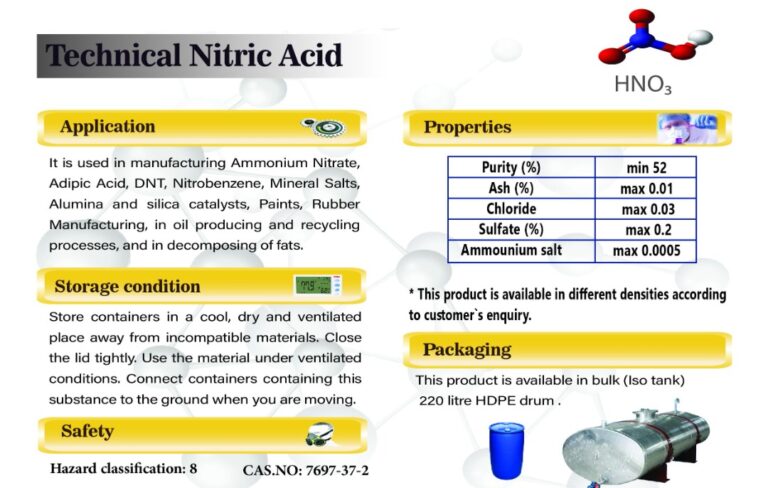
Nitric acid is used in the production of ammonium nitrate, adipic acid, DNT, nitrobenzene, inorganic salts, alumina and silica catalysts, paints, rubber production, oil production process and its recovery, as well as in the decomposition of fats.
Due to the strong properties of nitric acid, it should be handled carefully and properly. This acid can be toxic and uncomfortable and should not come into contact with skin, eyes and breathing. In case of contact with skin or eyes, wash immediately with water and see a doctor if necessary.
You should also avoid contact with this acid in areas other than near well-ventilated areas such as laboratories and places with proper air flow.
Maintenance
Store the containers in a cool, dry and ventilated place and away from incompatible materials. Close the nitric acid lid tightly. Use the material under ventilated conditions. Connect containers containing this substance to the ground when moving.
Export examples of Sipan Holding Citric acid in a barrel Citric acid packaging