What is nitric acid?
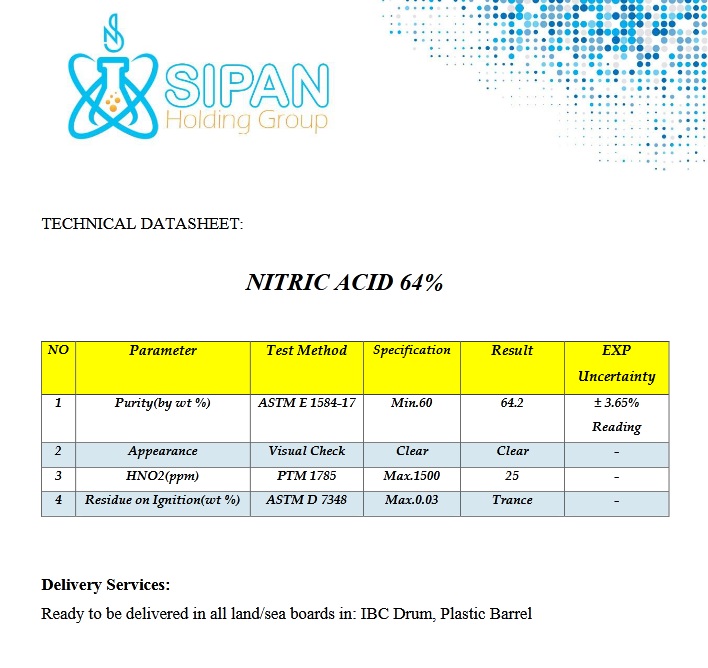
Nitric Acid 64% is a chemical compound represented by the formula HNO3. This acid is one of the strongest acids. It is widely used in the fields of chemistry and industry.
It is a colorless to yellow odorless liquid and is soluble in water. This matter is a strong oxidant and reacts with metals and other oxidizing agents such as ammonia (NH3). This reaction can produce toxic and exothermic gases such as nitrogen oxide (NOx).
The uses of Nitric Acid 64% in the industry:
Production of nitrogen fertilizers and explosives also paints and dyes and Production of plastics and nylons
Used in the chemical industry to produce a variety of chemical compounds
In case of using nitric acid, safety and necessary care should be strictly observed and direct contact with skin and eyes should be avoided.
Applications:
Industries and factories use nitric acid 64% (Nitric Acid) in the glass industry to make durable glass and increase the transparency of glass. In thermal processes (hardness and alumina). in the production of potassium nitrate. in the production of chemical fertilizers. in the production of limited medicines and in the preservation of meat. We use Nitric acid engraving metals and refining and extracting gold.
Where can I buy Nitric Acid 64%?
For many of you, dear producers and industrialists, this question is always important. “Where is the best place to buy affordable and high-tonnage products?”. Our suggestion to you, dear ones, is to contact the consultants of Sipan Company. which is a leading company in the field of chemical production and distribution.
Sipan Holding Company provides nitric acid at a very reasonable price and in any quantity according to your needs.
To know the price of each liter of nitric acid, contact the contact number of Sipan consultants to guide you.
Nitric acid 64% is one of the strong acids known in chemistry. This acid appears as a xylogram in its pure state. Also as a colorless and transparent liquid with a different smell. Nitric acid is soluble in water and is slightly vaporizable from water.
People know it as oxidizing agent and is widely used in the chemical industry. Especially in the production of chemicals, fertilizers, explosives and paints. This acid is also used as an antiseptic.
We can also find Various nitrates through the reaction of nitric acid with alkali metals or alkaline earth metals (such as sodium and potassium). For example, the reaction of nitric acid with ammonia can produce ammonium nitrate (NH4NO3).
Due to the strong oxidizing properties of nitric acid, Industries should use it with caution and safety principles in the environment. Direct contact with skin, eyes and mucous membrane can cause damage and burns. You should also avoid to expose it to heat or mix with flammable materials.

Transportation and loading of nitric acid:
The best way to transport nitric acid is in 220 kg barrels, because nitric acid is a very corrosive substance and carrying it in any other way is very risky. Sipan Holding, the only exporter of nitric acid, loads it at the dangerous goods area of Bandar Abbas.
For further information and purchasing inquiries, please contact the following number: